Question:
What
is used to make the dials and hands of watches glow in the dark?
Andrea Schwartz - Brisbane, Australia
Time in the dark
The problem
of telling the time in the dark dates back to very early days in the civilized world. The
invention of mechanical clocks equipped with repeater mechanisms, which chimed the hours
and quarter-hours on demand, brought a partial solution. Residents of those communities
rich enough to afford a belfry and clock were able to know the hour during the night with
reasonable precision provided that they paid attention to the number of bells chimed. In
other villages and hamlets, people generally had no choice but to use a sundial during the
daylight hours and a graduated candle at night, although the precision of the latter, a
fairly costly system, was not very good (see Figure 1).
In the 19th Century, the household clock became an indispensable object, accessible to a
greater number of people. Placed in an alcove, often in the bedroom, the Neuchâtel
pendulum clocks were equipped with mech-anisms that repeated the hours and quarter-hours
upon request. In this manner, it was possible to know the time to the nearest few minutes
even if you could not see the face of the clock. Before long, these timekeepers were
reduced to the dimensions of a pocket watch and remained very much in vogue until the use
of the wristwatch became widespread in the early 1920s.

Figure 1
What is light?
Light is a
form of energy. In order to create it, we must supply another form of energy. The two most
common ways for light to occur are incandescence and luminescence.
Incandescence is ‘hot light’, resulting from heat energy. The sun and other
stars in the universe shine because of incandescence. When you send electricity into the
filament of an ordinary light bulb, it glows ‘white hot’ filling the room with
incandescent light.
Luminescence, on the other hand, is ‘cold light’ from other sources of energy
occurring at normal and lower temperatures. In this phenomenon, an energy source causes an
electron of an atom to jump out of its lowest energy (ground) state into a higher energy
(excited) state. However, the electron prefers its ground state and when it falls back
into this position, it gives up energy in the form of light. There are several types of
luminescence, each named for the source of energy that causes it.
One very well known variety is bioluminescence. The most common examples are glow-worms
and fireflies, which use a chemical reaction to create their light. We can get a similar
effect in the laboratory by mixing together the appropriate compounds, but when the
chemical interaction ceases, the luminescence stops. Living organisms have the ability to
constantly renew the el-ements that cause bioluminescence. Obviously, we cannot do this
for the hands and dials of a watch. Therefore we need to look at two other processes for
lighting up the darkness: photoluminescence and radioluminescence.
Photoluminescence
In
photoluminescence, the energy is supplied by electromagnetic radiation, such as light. A
photoluminescent material absorbs light for a significant period of time and then
generally gives off light of a frequency lower than that of the absorbed light.
This phenomenon was known already in the 10th Century when Japanese painters used lacquers
prepared from photoluminescent oyster shells. One story tells of an artist who painted a
landscape scene depicting a bull that magically appeared in darkness but disappeared
during daylight. The animal was painted using a varnish that was the same colour as the
background, but made from a lacquer composed of ground oyster shells.
The first synthetic luminescent material appeared in Italy in the 17th Century, under the
names of ‘Stone of Bologna’ and ‘Sponge of Light’. It was composed of
the compound barium sulfide. By the end of the 19th Century, Swiss watchmakers began
treating the dials of timepieces with a natural luminescent paint created using the same
technique as the early Japanese artists.
The main drawback in using photo-luminescent material in watches is that the luminescence
diminishes rapidly and totally disappears after a few hours. This effect is called
‘decay’ and still exists today, although to a lesser degree, in material used as
a substitute for the more effective radioluminescent substances.
Radioluminescence
Radioluminescence
is produced by nuclear radiation. Gamma and X-rays or alpha and beta particles are used to
excite the electrons in a radioluminesc-ent compound such as zinc sulfide. This type of
radioluminescent material has been used in watches since before 1920 and up until a few
years ago. The dials kept their light emitting properties for a long time because they
were painted with a mixture of zinc sulfide and the radiation source. In many cases, the
radioactive source was a small quantity of thorium, promethium-147 or radium-226.
The French chemist, Marie Curie (1867-1934), discovered radium, at the expense of her own
health. At that time, no one was aware of the dangers of handling even small quantities of
this fascinating new material. Because of her research, she developed eczema and later
died of leukaemia, most likely caused from the exposure to this radioactive substance. For
many years, these elements were not considered dangerous to health or to the environment.
With the exception of a few nuclear physicists, the general population was unaware of the
dangers of radioactivity until the bombs exploded over Nagasaki and Hiroshima during World
War II. In fact, even as late as 1945, radioactivity was considered to be beneficial.
To cite just one example, in Germany in 1936, a rehabilitation clinic was built inside a
former salt mine saturated with the radioactive gas radon. Today, people living in
radon-rich areas have to use special pumps to remove this toxic gas from the basements of
their homes.
Even after WWII as people began to understand more about radioactivity’s harmful
effects, they could still buy alarm clocks with radium-painted dials that would make a
Geiger counter go crazy. There was also the example of a watch salesman who tried carrying
a valise full of chronographs and watches with luminous dials through an airport security
checkpoint in New York. The high levels of radioactivity given off caused the alarms to
sound and the bewildered soul was arrested, handcuffed and led away. While he was not
charged with any wrongdoing, this incident did a lot to discredit the use of
radioluminescent material in watches.
Enter tritium
The next
substance used to produce luminous watch dials and hands was tritium, a radioactive
isotope of hydrogen with an atomic mass of about 3. In the case of this element, the
radioactivity is composed entirely of beta par-ticles that are nearly completely absorbed
by the watch crystal or glass covering the dial. The tritium used in watches today is in
compliance with the international Standards ISO 3157 and NIHS 97-10, which define the
acceptable minimum levels for the amount of luminescence required to see the watch dial in
the dark. Depending on the quality of the radioluminescent compound, it can conserve its
ability to luminescence for several years. The quality also influences the luminous
intensity (see Figure 2), which also depends on the surface and thickness of the deposit
(see Figure 3). The pigments of the natural yellow tint yield the best results.
Even though the radioactivity of tritium is much weaker than the isotopes discussed
earlier, the handling of this product still requires special precautions. All workbenches
must be equipped with a vacuum hood to remove any harmful vapours and small particles.
Because they are mixed with the tritium, the radioluminescent materials are also
considered as radioactive sources during their application onto the watch and must be
handled with care.
Once inside the watch and under the glass, the radioluminescent materials pose no health
threat for the wearers. However, in the mind of the public, certain doubts persist,
causing some people to hesitate about the purchase of this type of timepiece.
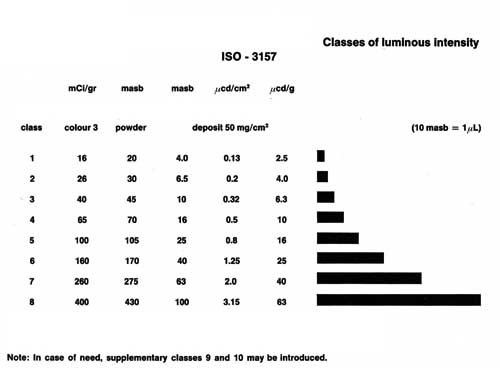
Figure 2
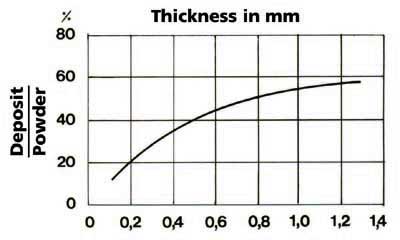
Figure 3
The new photoluminescence
The fear of
potential buyers, as well as the requirements that manufacturers furnish a certificate of
safety for products exported to the United States, has led Swiss watchmakers to re-examine
the use of photoluminescent material, which is exempt from the rules for radioactive
substances.
Japanese companies were the first to find substitute materials, which they named LumiNova
and LumiBrite. Although superior to the photoluminescent products used in the past, the
newer products still do not meet the minimum standards of visibility as stipulated in ISO
3157. A comparison of LumiNova and tritium compounds shows that, in the beginning, the
photoluminescent material is brighter, but that it decays at an exponential rate, crossing
the tritium curves at 40 and 120 minutes after activation in the two cases cited. It
continues to decrease rapidly and after a period of 3 to 6 hours, the luminous intensity
is very low (see Figure 4).
Another major disadvantage of this new compound is the necessity to ‘recharge’
it by exposing it to sunlight or a powerful lamp. If the watch is worn under the sleeve,
it will not get the required amount of light exposure and will not recharge itself
satisfac-torily. At the current time, production of this material in Switzerland is
carried out under a licensing agreement. The goal of the Swiss manufacturer, however, is
to create a new and better material that is not covered by patents and that would be
superior to tritium. A Swiss association for watchmaking research, the ASRH, has funded a
pro-ject carried out by the University of Lausanne and the Polytechnical Institute, the
EPFL, to research ways of improving photoluminescence in watches.
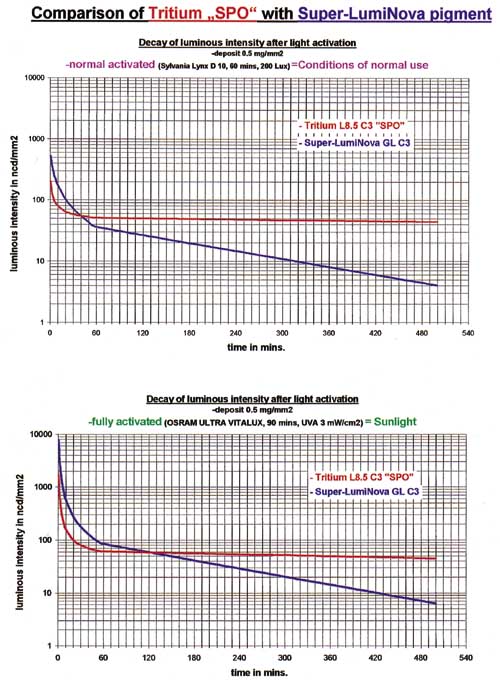
Figure 4
Safety concerns?
In the
meantime, the radioluminesc-ence produced by tritium is still the best solution to making
watches visible in the dark. Studies have shown that wearers need not fear the levels of
radioactivity given off by their luminous timepieces. An article in the British medical
journal, ‘The Lancet’ (Volume 343, No. 8889 January 8, 1994), compares the
annual dose of radioactive radiation absorbed through the skin of the wearer of a luminous
dial plastic watch with the total annual dose received from all sources. The radiation
dose is expressed in units called microsieverts. This measures the effective dose, taking
into account the type of radiation and the particular part of the body being irradiated.
According to this study, a plastic watch gives off an annual effective dose of 4
microsieverts. A chest X-ray exposes a person to a dose of 70 microsieverts. The average
annual dose received from natural background radiation is about 2100 microsieverts. This
clearly demonstrates that the radiation exposure from a plastic watch is negli-gible and
does not present
a health hazard.
|

 PRECISION WATCH REPAIR BY CHARLIE SQUIRES
PRECISION WATCH REPAIR BY CHARLIE SQUIRES